Indications for use
Bronchial asthma
The medicine is available in different forms and can be used to eliminate the symptoms of many diseases. As a medicine for inhalation, it is prescribed for bronchial asthma and COPD. In the first case, Fluticasone is the basis of anti-inflammatory therapy and can be prescribed even to a child over 1 year old. For COPD, the drug complements the main treatment and is used only by adults.
To relieve the symptoms of allergic rhinitis and hay fever, adults and preschool children are prescribed intranasal use. The product allows you to quickly cope with nasal congestion and runny nose, sneezing, watery eyes, and itching. The drug is highly effective against various dermatoses, dermatitis, psoriasis, lichen and other skin reactions. For treatment in this case, the drug is prescribed locally. The release form, course duration, and dosage depend on the specific diagnosis and condition of the patient.
Contraindications
The drug Fluticasone is based on steroid hormones, so the drug has a number of serious contraindications that you need to be aware of before you start taking it. In addition to high sensitivity to the components of the drug, dangerous contraindications are:
- Acute bronchospasm.
- Status asthmaticus (except in cases where fluticasone is used as an additional therapy).
- Children's age (for different forms of release it ranges from 6 months to preschool age).
- Nose trauma or recent surgery.
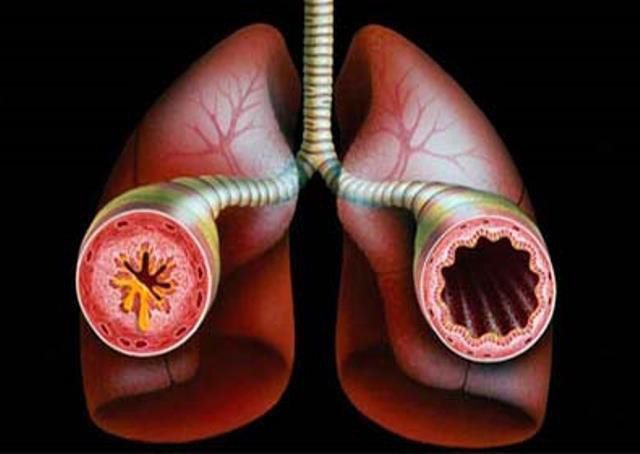
Acute bronchospasm - Bacterial, viral or fungal skin lesions (for local treatment of the skin).
- Perioral dermatitis.
- Perianal and genital itching.
The listed diseases are absolute contraindications, but there are also other restrictions that the attending physician should be aware of before prescribing treatment with Fluticasone. Among them: liver cirrhosis, glaucoma, cataracts, hypothyroidism, various infections (including HIV), osteoporosis, pregnancy and the subsequent period of breastfeeding, old age. The decision on the possibility of prescribing Fluticasone in the presence of such diagnoses is made by the doctor on an individual basis.
Fluticasone - instructions, composition, dosage, side effects of use
Fluticasone
Fluticasone Cutivate (UK), Flixonase (Spain), Flixotide (UK), Flixotide (France)
Inhaled glucocorticoids. ATC code R03A K06. Release form of the drug Aerosol for inhalation doses. 0.125 mg/dose; 0.25 mg/dose; 0.05 mg/dose Cream external. 0.05% Ointment external. 0.005% Port. for inhalation doses. 0.25 mg/dose; 0.5 mg/dose Spray called. 50 mcg/dose Feature of the chemical structure Derivative of cyclopentaneperhydrophenanthrene. Mechanism of action See "Beclomethasone". Main effects • In recommended doses, Fluticasone has a pronounced anti-inflammatory, immunosuppressive and antiallergic effect, which helps reduce the severity of symptoms, frequency and severity of exacerbations of diseases accompanied by airway obstruction (bronchial asthma, chronic bronchitis, emphysema). See also "Beclomethasone". • In therapeutic doses, it has virtually no systemic effect, including effects on the hypothalamic-pituitary-adrenal system. • Restores the patient's response to bronchodilators, allowing them to reduce the frequency of their use. • The therapeutic effect after inhalation use begins within 24 hours, reaches a maximum within 1-2 weeks or more after the start of treatment and persists for several days after discontinuation. Pharmacokinetics After inhalation administration, absolute bioavailability is 13.5% (powder) 30% (aerosol). Absorption occurs predominantly in the lungs. Part of the inhaled dose can be swallowed, but its systemic effect is minimal due to the low solubility of the drug in water and intensive metabolism during the first passage through the liver (bioavailability after absorption from the gastrointestinal tract is less than 1%). There is a direct relationship between the size of the inhaled dose and the severity of the systemic effect. Binds to plasma proteins by 91%. The volume of distribution is about 300 l (4.2 l/kg). Total clearance - 1.15 l/min (renal clearance - less than 0.2%). Metabolized in the liver with the participation of the CYP3A4 enzyme of the cytochrome P450 system with the formation of a weak or inactive metabolite. T1/2 ~ 8 hours. Excreted through the intestines and kidneys (less than 5% is excreted in the urine as a metabolite). When administered intranasally in doses of up to 1 mg, the plasma concentration is very low and approaches the sensitivity threshold of the method (0.05 ng/ml). When used externally, it is practically not subject to systemic absorption; The drug absorbed through the skin is intensively metabolized, quickly inactivated and excreted mainly in bile within 48 hours. It does not accumulate in tissues and does not bind to melanin.
Indications for use. • bronchial asthma (basic therapy, including in severe cases of the disease and dependence on systemic glucocorticoids). • COPD. • With intranasal administration: allergic rhinitis (seasonal and year-round) - prevention and treatment. Other indications: • eczema, nodular prurigo, psoriasis (except widespread plaque), neurodermatoses, lichen planus, contact dermatitis, discoid lupus erythematosus, generalized erythroderma (as an adjunct to GCS therapy for systemic use), insect bites, miliaria erythematosus, seborrheic dermatitis. Method of administration and dosage: The drug should be used regularly by inhalation even in the absence of symptoms of the disease. The frequency of inhalations is 2 times a day. The therapeutic effect usually occurs 4-7 days after the start of treatment. In patients who have not previously taken inhaled glucocorticoids, improvement may be observed within 24 hours after starting inhalations. Depending on the individual response to treatment, the initial dose can be increased until the effect appears or decreased to the minimum effective dose. The initial dose of fluticasone propionate corresponds to half the daily dose of beclomethasone dipropionate. The drug can be administered through a spacer (for example, Volumatic). For adults and adolescents over 16 years of age, the initial dose for mild bronchial asthma is 100-250 mcg 2 times a day; moderate severity - 250-500 mcg 2 times / day, severe - 500-1000 mcg 2 times / day. Children over 4 years old are prescribed 50-100 mcg 2 times a day. Dose for children 1-4 years old - 100 mcg 2 times a day (only in the form of an aerosol dosed for inhalation). Young children require higher doses compared to older children (due to difficult delivery of the drug during inhalation - smaller bronchial lumen, use of spacer, intense nasal breathing in young children). The drug is especially indicated for young children with severe bronchial asthma and is administered using an inhaler through a spacer with a face mask (for example, Babyhaler). For the treatment of chronic obstructive pulmonary disease, adults are prescribed 500 mcg per day. Patients with impaired liver and/or kidney function, as well as elderly people, do not require dose adjustment. Intranasally. To achieve the full therapeutic effect, the drug should be used regularly. Adults and children 12 years of age and older are prescribed 100 mcg in each nasal passage 1 time per day (preferably in the morning), in some cases - 2 times per day. The maximum daily dose should not exceed 200 mcg in each nasal passage. Elderly patients do not require dosage adjustment. Children aged 4-11 years are prescribed 50 mcg in each nasal passage 1 time per day. The maximum daily dose is 100 mcg in each nasal passage. The maximum therapeutic effect appears after 3-4 days. Contraindications • Hypersensitivity. • Acute bronchospasm. • Status asthmaticus (as a first-line remedy). • Bronchitis of non-asthmatic nature. • Pulmonary tuberculosis (for inhalation). • Candidiasis of the upper respiratory tract. • Children's age (up to 1 year - for inhalation, up to 4 years - for intranasal use). • For external use - rosacea, acne vulgaris, perioral dermatitis, perianal and genital itching, primary skin lesions of bacterial, viral and fungal etiology, children's age (for ointment - up to 1 year, for cream - up to 6 months). Cautions, monitoring of therapy • Not intended for the relief of attacks of bronchial asthma. • Aerosol for inhalation - dosage regimen for children under 12 years of age has not been established. Powder for inhalation - dosage regimen for children under 4 years of age has not been established. • According to the FDA-approved categories of effect on the fetus, it is classified as Category C (experimental studies of the effect on reproductive function have not revealed an adverse effect on the fetus, and adequate and well-controlled studies in pregnant women have not been conducted. The potential benefits associated with the use of drugs in pregnant women may justify its use despite the possible risks). • Fluticasone should be used with caution concomitantly with inhibitors of the CYP3A4 enzyme. • The patient must be taught how to use the inhaler correctly, and also warned about the need to immediately consult a doctor if there is a decrease in the effectiveness of short-acting bronchodilators or more frequent use of the inhaler is required. • An increased need for inhaled short-acting β2-adrenergic receptor agonists indicates a worsening of the disease. In such cases, it is recommended to reconsider the patient's treatment plan. A sudden and progressive deterioration in the course of bronchial asthma can pose a threat to the patient's life, so it is urgent to resolve the issue of increasing the dose of GCS. • In case of severe exacerbation of bronchial asthma or insufficient effectiveness of the therapy, the dose of inhaled fluticasone should be increased, and if necessary, systemic administration of GCS and/or if an infection develops, an antimicrobial agent should be prescribed. • To prevent hoarseness and the development of candidiasis, you should rinse the mouth and pharynx after inhalation. If necessary, local antifungal therapy can be prescribed throughout the entire treatment period. • If paradoxical bronchospasm develops, inhalation should be stopped immediately, the patient's condition assessed, the necessary examination carried out and, if necessary, other medications prescribed. Paradoxical bronchospasm should be treated immediately with a fast-acting inhaled bronchodilator. • With long-term use of any inhaled corticosteroids, especially in high doses, systemic effects may be observed, but the likelihood of their development is much lower than when taking corticosteroids orally. Considering this, when a therapeutic effect is achieved, it is recommended to reduce the dose of fluticasone to the minimum effective. • When carrying out resuscitation or surgical interventions, the degree of adrenal insufficiency must be determined. In such stressful situations, one should always take into account possible adrenal insufficiency and, if necessary, additionally prescribe corticosteroids. Due to the possibility of latent adrenal insufficiency, special care should be taken (regularly assess the function of the adrenal cortex) when transferring patients who have taken oral corticosteroids to treatment with inhaled drugs. The withdrawal of systemic glucocorticoids against the background of inhaled fluticasone should be carried out gradually. Patients should carry a card with them indicating that they may need to take additional corticosteroids during stress (for example, with the development of severe concomitant diseases, surgery, trauma, etc.). • In rare cases, when transferring patients from taking systemic glucocorticoids to inhaled therapy, conditions accompanied by hypereosinophilia (for example, Churg-Strauss syndrome) may appear. As a rule, this occurs during dose reduction or withdrawal of systemic glucocorticoids, but a direct relationship has not been established. • When transferring patients from taking systemic glucocorticoids to inhaled therapy, allergic reactions (eg, allergic rhinitis, eczema) that were previously suppressed by systemic drugs may also occur. In such situations, it is recommended to carry out symptomatic treatment with antihistamines and/or topical drugs, including corticosteroids for topical use. • The growth dynamics of children receiving inhaled glucocorticoids for a long time should be regularly monitored. • As with most aerosol inhalation products, the effect decreases as the can cools. • The rotadisk can be stored in a disc haler, but the cell should be pierced only immediately before inhalation. • Transferring patients suffering from hormone-dependent bronchial asthma from systemic glucocorticoids to inhaled fluticasone requires special attention, since restoration of adrenal function takes a long time. Adrenal function should be regularly monitored and caution should be exercised when reducing the dose of systemic glucocorticoids. • A gradual reduction in the dose of systemic glucocorticoids can begin approximately one week after fluticasone administration. With a maintenance dose of prednisolone (or other corticosteroids at an equivalent dose) of less than 10 mg/day, dose reduction should not exceed 1 mg/day and should be carried out at intervals of at least 1 week. With a maintenance dose of prednisolone over 10 mg/day (calculated per day) - in large doses, also at intervals of at least 1 week. • Some patients, during the period of reducing the dose of systemic glucocorticoids, complain of general malaise against the background of stabilization or even improvement of respiratory function. If there are no objective signs of adrenal insufficiency, patients should be encouraged to continue switching to inhaled glucocorticoids and gradually withdrawing systemic glucocorticoids. Prescribe with caution: • for liver cirrhosis; • for glaucoma; • for hypothyroidism; • for systemic infections (bacterial, fungal, parasitic, viral); • for osteoporosis; • for pulmonary tuberculosis (intranasal); • during pregnancy; • during lactation. Side effects From the respiratory and digestive systems: • candidiasis of the mucous membrane of the oral cavity and pharynx; • hoarse voice; • nasal congestion; • upper respiratory tract infections; • bronchitis; • cough; • sneezing; • dyspnea; • paradoxical bronchospasm; • nausea; • vomit; • discomfort or pain in the stomach area; • diarrhea; • with intranasal use - dryness or irritation of the nasal mucosa, nasopharynx, burning, unpleasant taste and smell, nosebleeds, pharyngitis, cough, rhinorrhea, nasal congestion, flu-like symptoms; in isolated cases - perforation of the nasal septum, especially with a history of surgical interventions in the nasal cavity. From the side of the central nervous system: • headache; • dizziness; • weakness; • malaise; • insomnia. Other effects: • systemic side effects: with long-term use in high doses, concomitant or previous systemic use of GCS, in rare cases, decreased function of the adrenal cortex, osteoporosis, growth retardation in children, cataracts, increased IOP are observed; • intranasal administration of 2 mg 2 times a day to healthy volunteers for 7 days did not reveal any effect on the hypothalamic-pituitary-adrenal system; • for external use - local reactions: dryness, irritation, burning and itching at the site of application, for long-term use - skin atrophy, stretch marks, dilatation of superficial blood vessels, hypertrichosis, hypopigmentation. In isolated cases or rarely - allergic contact dermatitis, development of secondary infections (especially when using occlusive dressings and application to the area of skin folds), exacerbation of symptoms of dermatosis, during the treatment of psoriasis (or when withdrawing GCS), the development of a pustular form of the disease is possible, with long-term use in at high doses (or in the presence of factors that increase absorption), systemic absorption of the drug is possible with the development of systemic side effects, including symptomatic hypercortisolism (more often in infants); • rarely - allergic reactions. Exceeding the permissible dose of the drug (overdose) Symptoms: in case of acute overdose, a temporary decrease in the function of the adrenal cortex is possible, in case of chronic overdose - persistent suppression of the adrenal cortex. No symptoms of acute or chronic overdose of the drug with intranasal use have been recorded. Treatment: in case of acute overdose, emergency treatment is not required, since the function of the adrenal cortex is restored within a few days. In case of chronic overdose, it is recommended to monitor the reserve function of the adrenal cortex. Treatment should be continued in doses sufficient to maintain the therapeutic effect.
Dicloberl 50 suppositories - instructions, composition, dosage, side effects of use
Interaction
Groups and drugs Result CYP3A4 inhibitors (including ketoconazole, ritonavir The systemic effect of fluticasone may be enhanced due to an increase in its concentration in the blood plasma Other drugs Interaction with other drugs is unlikely, since inhalation, intranasal and cutaneous use of fluticasone concentrations in plasma are very low.
Sources of information: rsml.med.by, mednet.by, drugs.com, webmd.com.
Composition of the drug
The main active ingredient in the drug is fluticasone propionate. It belongs to the group of corticosteroids that affect carbohydrate metabolism. The substance has a strong anti-inflammatory effect and practically does not inhibit brain function, which is typical for similar medications. The noticeable effect of taking the drug occurs a couple of hours after the first dose and persists throughout the day.
As additional components, the medical product in the form of a spray contains:
Composition of the drug
- Benzalkonium chloride is an antiseptic that can occasionally cause an allergic reaction.
- Polysorbate – added as a solvent.
- Phenylethanol – added as a preservative.
- Dextrose is a sweetener.
Fluticasone cream and ointment contain:
- Liquid paraffin.
- Propylene glycol.
- Cetostearyl alcohol.
- Microcrystalline wax and some other chemicals in trace quantities.
Almost none of the substances that make up Fluticasone can have a negative effect on the body, but if a reaction to one of them has been noticed in the past, you should consult your doctor about further use.
Mechanism of action
The principle of action of Fluticasone is based on its active contact with glucocorticosteroid receptors. The medicine weakens the proliferation of mast cells, eosinophils, lymphocytes, neutrophils and other cells. In addition, Fluticasone reduces the amount of histamine, prostaglandins, and cytokines, which reduces a pronounced allergic reaction.
The inflammatory process is noticeably reduced within two hours after the start of use. Symptoms disappear completely or become less noticeable, which improves the quality of life of the person suffering from the disease. The resulting effect lasts for a day. Suppression of the hypothalamic-pituitary-adrenal system does not occur even with intranasal use of the drug.
Fluticasone
International name of the medicinal substance:
Fluticasone The list of drugs containing the active substance Fluticasone is given after the description.
Pharmacological action:
GCS for local use (inhalation and intranasal);
has anti-edematous, anti-inflammatory and anti-allergic effects. Restores the patient's response to bronchodilators, allowing to reduce the frequency of their use. Suppresses the proliferation of mast cells, eosinophils, lymphocytes, macrophages, neutrophils, reduces the production and release of inflammatory mediators and other biologically active substances (histamine, Pg, leukotrienes, cytokines). Pharmacokinetics:
When administered intranasally in doses of up to 1 mg, the plasma concentration is very low and approaches the sensitivity threshold of the method (0.05 ng/ml).
Indications:
Allergic rhinitis (seasonal and year-round) - prevention and treatment.
Contraindications:
Hypersensitivity.
Side effects:
Local reactions (very rare): dryness and irritation of the mucous membrane of the nasopharynx, burning, unpleasant taste and smell, nosebleeds, nasal congestion;
in isolated cases - perforation of the nasal septum, especially with a history of surgical interventions in the nasal cavity. When administered intranasally to healthy individuals 2 mg 2 times a day for 7 days, no effect on the function of the hypothalamic-pituitary-adrenal system was found. Special instructions:
With long-term use, regular monitoring of the function of the adrenal cortex is necessary.
To relieve ophthalmological manifestations of allergic diseases, even against the background of successful treatment of seasonal allergic rhinitis, additional therapy may be required. Upper respiratory tract infections are not a contraindication for use. Caution should be used after systemic corticosteroids, especially in cases where suppression of adrenal function is expected. Preparations containing the active ingredient Fluticasone:
Cutivate, Nazarel, Sinoflurin, FeniVate, Flixonase, Flixotide
The information provided in this section is intended for medical and pharmaceutical professionals and should not be used for self-medication. The information is provided for informational purposes only and cannot be considered official.
Release forms
In modern pharmacology, Fluticasone is produced in 4 forms, each of which is best suited for the treatment of specific groups of diseases. The doctor may prescribe medicine in the form of:
Fluticasone nasal spray
- creams for external use;
- ointments for external use;
- nasal spray in bottles;
- powder for inhalation;
- aerosol for inhalation.
The first two forms are used to eliminate symptoms of skin diseases, allergic reactions, and relieve itching after insect bites. The rest are for bronchial asthma, COPD and other problems of the respiratory system.
Dosage and method of administration
Directions for use and dosage:
Intranasal, local, inhalation. Nasal spray - in the morning, 100 mcg (children under 12 years old - 50 mcg) in each nostril; aerosol for inhalation - for mild, moderate and severe bronchial asthma 2 times a day, 100-250 mcg, 250-500 mcg and 500 mcg, respectively (children and adolescents under 16 years old - 50-100 mcg); ointment and cream are applied in a thin layer to the affected area of skin 2 times a day. Precautions: Prescribe with caution after systemic use of glucocorticoids, especially in case of impaired adrenal function; inhalation aerosol - for pulmonary tuberculosis. The nasal spray is recommended to be used at regular intervals; treatment with an inhalation aerosol should not be stopped abruptly; before the procedure, it is advisable to inhale a short-acting beta2-adrenergic receptor agonist, and after it, rinse the mouth. It is necessary to avoid getting the cream or ointment into the eyes; before applying a new bandage, the skin is thoroughly cleaned.
How to take it correctly
Features of taking a medication depend on its form and the disease for which it is used. Fluticasone in the form of an aerosol and powder for inhalation is prescribed when it is necessary to treat chronic bronchial obstruction. The product should be used regularly, even if there are no symptoms of the disease at the moment.

Using an inhaler
Inhalations must be carried out twice a day. The aerosol is used independently, so you can take it with you. For inhalations with powder, you will need an inhaler, and if the treatment is prescribed to a child, you will also need a spacer. The therapeutic effect occurs a few days after regular use of the drug begins. The dosage depends on the patient’s age and the degree of the disease. It is selected individually by the attending physician, taking into account the medical history.
The drug Fluticasone is not suitable for relieving an acute attack of bronchial asthma. In case of a sharp deterioration of the condition, the patient requires other medical care.
A nasal spray is prescribed when symptoms of allergic rhinitis of various etiologies occur. It is recommended to use the drug regularly. For preventive purposes, it is enough to use the product once a day; in case of an acute allergic reaction, the dosage can be increased to twice a day.

Using ointment
Fluticasone in the form of ointment and cream can be applied not only to treat the acute phase of the disease, but also to reduce the number and frequency of relapses. The medication is applied directly to the affected area of the skin in a thin layer once or twice a day. The course of treatment for the acute stage lasts two weeks. Then the number of applications is reduced to 1 time per week on those areas of the skin that were previously damaged.
To learn more about how to use Fluticasone, you must seek help from the specialist who prescribed the treatment and read the detailed instructions for use. This will avoid the development of unwanted side effects.
Fluticasone (fluticasone propionate)
Asthma. The lowest effective dose should be used. In the case of doses > 1000 mcg per day, it is recommended to use an inhalation chamber. Adults and children over 16 years of age. The drug Flixotide is an aerosol, powder for inhalation. Mild asthma - 200-500 mcg per day in 2 divided doses, moderate asthma - 500-1000 mcg per day in 2 divided doses, severe asthma - 1000-2000 mcg per day in 2 divided doses. The drug Flutixone is a powder for inhalation in capsules. Mild asthma - 250 mcg per day in 2 divided doses, moderate asthma - 250-500 mcg per day in 2 divided doses, severe asthma - 500-1000 mcg per day in 2 divided doses. Children over 4 years of age (aerosol, powder for inhalation). 100-200 mcg per day in 2 divided doses. Children aged 1-4 years (aerosol, apply using an inhalation chamber with a face mask). 100-200 mcg per day in 2 divided doses. In the form of a suspension for inhalation using a nebulizer in patients over 16 years of age 1-4 mg per day in 2 divided doses, in children over 4 years of age 2 mg per day in 2 divided doses. COPD Adults. The drug Flixotide is an aerosol, powder for inhalation. 1000 mcg per day in 2 divided doses. The drug Flutixone is a powder for inhalation in capsules. 500 mcg per day in 2 divided doses. Allergic rhinitis. In the form of a nasal spray. Fluticasone propionate. Adults and children over 12 years of age: 1 time per day (preferably in the morning) 2 doses (1 dose = 50 mg) in each nostril or, if necessary, 2 times a day, no more than 4 doses per day in each nostril. Children aged 4-11 years: 1 dose per day in each nostril or, if necessary, 2 times a day, maximum 2 doses per day in each nostril. After improvement, the dose should be reduced to the minimum effective dose. Fluticasone furoate. Adults and children over 12 years old. At the beginning of therapy, 2 doses in each nostril 1 time per day (total daily dose 110 mcg), and after achieving optimal control of symptoms, the dose is reduced to 1 dose in each nostril 1 time per day. Children aged 6-11 years. 1 dose (27.5 mcg) in each nostril 1 time per day (total daily dose 55 mcg), if necessary, the dose can be increased to 2 doses in each nostril 1 time per day (total daily dose 110 mcg), and after achieving For optimal control of symptoms, the dose should be reduced. In the form of nasal drops in disposable containers. Adults and children over 16 years of age - approximately 6 drops (half the contents of the container, approx. 200 mcg) in each nostril 1-2 times a day for several weeks. If no improvement is observed after 4-6 weeks of use, the need to change the method of treatment should be considered. Topically on the skin. It is necessary to apply ointment or cream to the affected areas of the skin 2 times a day until improvement is achieved, then the drug should be used once a day or every other day, or use a drug that has a weaker effect.
Analogs
The official medical name of the drug is fluticasone propionate. This medical product is hidden under several brand names, produced in different forms and dosages. Among the most famous analogues are:

Flixonase spray
- Nazarel, Flixonase, Avamis - are available in the form of a nasal spray for the treatment of rhinitis.
- Cutivate, Fluderm - available in the form of cream and ointment.
- Flixotide is available in the form of an aerosol for inhalation.
- Flutixone is available in powder form for inhalation.
In addition to synonymous drugs, Fluticasone has substitutes among drugs based on other active corticosteroids, including Betamethasone, Budesonide, Halomethasone. It is not recommended to replace one medication with another without prior consultation with your doctor, since they have different effects on the body, and therefore have other contraindications and adverse reactions.
Adverse reactions
The use of Fluticasone is associated with the development of a large number of dangerous side effects. Among the most common are:
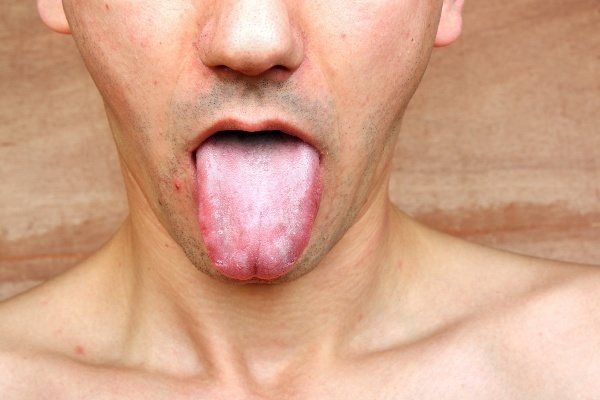
Candidal stomatitis
- Candidal stomatitis.
- Pneumonia (common in patients with COPD).
- Headache.
- Taste disturbances and changes in smells.
- Bruising.
- Itching (when using ointment or cream).
If one or more adverse reactions are detected, it is recommended to seek help from a medical facility, where they will help eliminate unpleasant symptoms and review the course of treatment.
If the use of Fluticasone continues for a long time, it is necessary to conduct additional examinations to understand whether dysfunction of the hypothalamic-pituitary-adrenal system has begun, especially if the treatment concerns a child. One severe consequence to watch out for in children may be slower growth.
https://youtu.be/X-MA7HTR9k8
The dosage and frequency of use should not be exceeded, so as not to lead to a return of symptoms of the disease or to an overdose. It is also not recommended to abruptly stop using Fluticasone. A specialist will tell you when to finish the course of treatment. When used correctly, Fluticasone is absolutely safe and can be used for a long period of time without causing any complications.
Side effects
- dryness and burning of the mucous membrane of the nasal and oral cavity;
- change in smell and taste;
- nosebleeds;
- headache;
- rash, itching, swelling of the skin;
- bronchospasm;
- anaphylactic shock;
- angioedema;
- nasal congestion;
- perforation of the nasal septum;
- fungal infection of the nasal and oral cavity;
- hoarseness of voice;
- cataract (clouding of the lens);
- delayed growth and development in children;
- atrophic changes in the skin.